Aluminium is Highly Reactive Metal
The compounds formed by highly reactive non-metals with highly reactive metals are generally ionic because of large differences in their electronegativities. Aluminiums free metal cation Alaq3 is highly biologically reactive and biologically available aluminium is non-essential and essentially toxic.
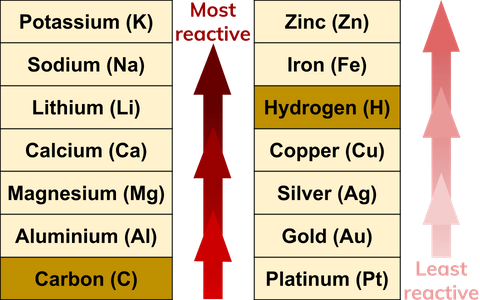
What Are Reactivity Series Definition From Seneca Learning
This is because its protective aluminium oxide layer makes it appear to be less.

. Boron is a typical non-metal aluminium is a metal but shows many chemical similarities to boron and gallium indium thallium and nihonium are almost. Corundum Al 2 O 3. Bauxite is a mixture of aluminium hydroxide iron oxide titanium dioxide and kaolinite.
O 2 is easily dismutated to H 2 O 2 either nonenzymatically or by superoxide dismutase. Stepwise monovalent reduction of O 2 leads to formation of O 2 H 2 O 2 and OH whereas energy transfer to O 2 leads to formation of 1 O 2. The important ores of aluminium are.
In nature aluminium is found in the form of its oxide in its ores. Activation of O 2 occurs by two different mechanisms. Cryolite Na 3 AlF 6.
Aluminium is a highly reactive metal belonging to the IIIA group of the periodic table. Once it gains entry into the cell Hg 0 is oxidized and becomes highly reactive Hg 2. On the other hand.
The elemental vapor is highly lipophilic and is effectively absorbed through the lungs and tissues lining the mouth. Aluminium production is highly energy-consuming and so the producers tend to locate. It is often protected by a layer of inert transparent Aluminium oxide Al 2 O 3 that forms rapidly in the air but is found to be highly reactive in nature.
Rusting is an oxidation reaction. Biologically reactive aluminium is present throughout the human body and while rarely it can be acutely toxic much less is understood about chronic aluminium intoxication. Aluminium forms amphoteric oxides that is it shows both acidic and basic character.
Aluminium doesnt occur as a isolated ore its too reactive with other compounds but as one combined with other minerals chief of which is bauxite. Resulting in the final aluminium metal. Bauxite Al 2 O 32H 2 O.
Dimethylmercury and tetraethyllead Copper fuel cell catalysts eg CuZnOAl 2 O 3. Read more about electron configuration here. Schematic representation of generation of reactive oxygen species ROS in plants.
A more reactive metal will displace a less reactive metal from a compound. The aluminium trialkyls and triaryls are reactive volatile and colorless liquids or low-melting solids. Bauxite is mined from open mines from locations mainly in a wide belt around the equator.
Other known oxidation states are 2 and 1. They catch fire spontaneously in air and react with water thus necessitating precautions when handling them. After Hg 0 enters the blood it rapidly passes through cell membranes which include both the blood-brain barrier and the placental barrier.
Metal hydrides sodium hydride lithium aluminium hydride uranium trihydride Partially or fully alkylated derivatives of metal and nonmetal hydrides diethylaluminium hydride trimethylaluminium triethylaluminium butyllithium with a few exceptions ie.
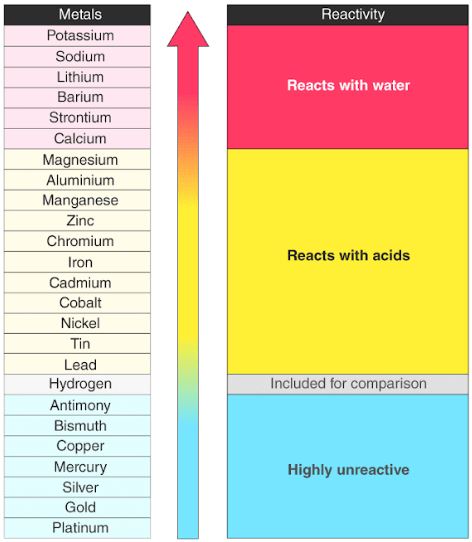
Reactivity Series Reactivity Of Metals Chart Features Uses

Reactivity Series Of Metals Toppr Bytes

Aluminium Is Highly Reactive Metal Yet It Is Used To Make Utensils Why Brainly In
Which Metal Is More Reactive Aluminium Or Iron And Why Quora

The Metal Reactivity Series Compound Interest
0 Response to "Aluminium is Highly Reactive Metal"
Post a Comment